ABSTRACT
Silver tarnishing is a problem experienced by all owners, curators and conservators of silver collections. The black silver tarnish (silver sulphide) which forms as a result of the reaction of silver with sulphur-containing gases in the atmosphere detracts from the beauty of silver artefacts. In addition, regular cleaning can result in the loss of fine detail from the surface of unique works of art. In some cases, for example silver threads on historic textiles or daguerretype photographs, safe and effective tarnish removal is particularly difficult to accomplish.
In order to achieve superior protection of museum, ecclesiastical or private silver collections from tarnishing, a 3-year research project, "SILPROT", was funded by the European Commission DG XII Environment Programme in 1998. By adopting an integrated, multidisciplinary approach, SILPROT is designed to provide a European solution to an international problem. The project team comprises specialists in environmental science, conservation research and absorbents for sulphur removal. The aims are to:
This paper will concentrate on the use of absorbents to remove sulphur gases from showcases as a means of preventative conservation. The synthesis and characterisation of transition metal based absorbents will be discussed briefly before discussing more fully the testing procedures used to evaluate the absorbents under laboratory conditions. Finally a comparison of the SILPROT absorbents with commercially available absorbents will be made indicating that the SILPROT absorbents are equal to- and in many cases superior to- the commercial absorbents tested (under the same laboratory conditions).
NOTES FROM SHEETS
SYNTHESIS
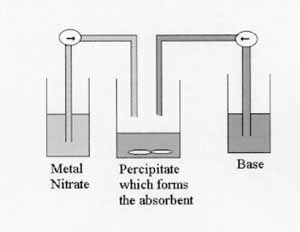
The absorbents are produced using the co-precipitation technique. This method allows total control of the synthesis conditions of the materials including the temperature, pH, concentration of the reactants and rate of reaction.
The initial materials that are produced are called precursors and although these can be used as absorbents themselves without further treatment, they are often calcined i.e. heated under flowing air to produce different materials called oxides. The precursors are usually coloured and indicate when they are exhausted by changing colour to black. The oxides are black but have higher surface areas and are usually better absorbents.

The co-precipitation technique as illustrated above can be used to control the synthesis of the absorbents.
Absorbent Testing
2% H2S passed over a 5cm3 absorbent bed and alkaline lead acetate solution used to detect breakthrough of H2S after absorbent failure. The H2S causes lead sulphide to precipitate out of the lead acetate solution and this can be monitored using a light gate as shown below.
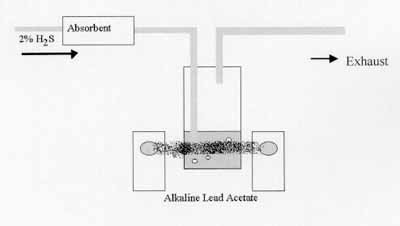
The absorbents can be pelletised for ease of use and are approximately 1000µm in size when tested.
Results
Selected results to illustrate the efficiency of the absorbents under laboratory test conditions:
| Absorbent | Breakthrough Time (mins) | % Conversion | % Reaction |
| SILPROT 1 | 110 | 23.33 | 82.50 |
| SILPROT 2 | 283 | 25.89 | 86.59 |
| SILPROT 3 | 340 | 46.29 | 109.05 |
| SILPROT 4 | 151 | 12.58 | 29.72 |
| SILPROT 5 | 88 | 20.67 | 84.52 |
| SILPROT 6 | 433 | 42.10 | 99.38 |
| SILPROT 7 | 58 | 6.86 | 16.14 |
| Commercial 1 | 7 | 0.66 | ------ |
| Commercial 2 | 29 | 2.31 | ------ |
| Commercial 3 | 7 | 0.40 | ------ |
| Commercial 4 | 8 | 0.49 | ------ |
| % Conversion = weight of H2S absorbed / weight of absorbent % Reaction = moles of H2S absorbed / moles of metal |
Conclusions
[ Page up ] [ IAP Group homepage ] [ Main IAQ in Museums homepage ] [ Search site ]
Indoor Air Quality in Museums and Archives
© May 11th, 2000